

![]()
CLINICAL AND PRECLINICAL PROGRAMS
CANCER IMMUNOTHERAPY
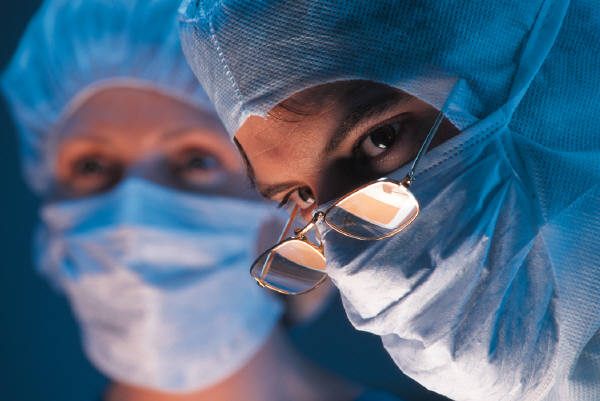
Immunotope’s immunotherapeutic products are designed to elicit long-lasting immunity in the patient to destroy tumor cells whenever and wherever they arise, even after distal invasion.
IMT-1012 Immunotherapeutic Vaccine Phase I Clinical Trial for Treatment of Advanced Stage Ovarian and Breast Cancer
A Phase I clinical trial with our lead product, the IMT-1012 Immunotherapeutic Vaccine, a 12 antigen composition has been completed and 7 antigens from IMT-1012 has been licensed to Immunovaccine Inc., Halifax, Canada and being evaluated in a phase I clinical study as DPX-0907 formulation.
Immunotope's IMT-1012 vaccine is the first immunotherapeutic treatment to combine twelve different novel antigens, each of which targets a separate, critical pathway known to be present in highly aggressive tumors, including:
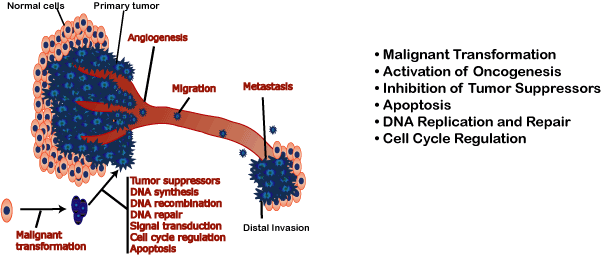
(Figure adapted from Chambers and Matrisian J Natl Cancer Inst. 1997:89:1260-70)
This multi-targeting strategy provides broad coverage to account for tumor complexity and heterogeneity, and significantly minimizes the potential for tumor escape.
Preclinical Development
Our R&D pipeline focuses on the characterization and preclinical validation of proprietary, indication-optimized panels of novel tumor signature antigens that target essential tumor pathways for lung, pancreatic and colon cancers. Immunotope’s products are intended for use in a therapeutic setting where it is essential to eradicate minimal residual disease after surgery and chemotherapy to prevent recurrence and metastasis.
A rapid timeline from discovery to human clinical testing can be achieved with antigens that are naturally present in the human body. Prior to human testing, in vitro validation studies are performed to verify that each antigen in the vaccine activates CTL to recognize and destroy a variety of tumor cells and do not destroy normal tissues. These minimal requirements for preclinical testing accelerate the timeline for research, development and Phase I/II clinical testing of Immunotope’s immunotherapeutics.
CANCER
DIAGNOSTICS
.....................................................................................................................................................................
Clinical and Preclinical Programs | Science & Technology | Careers
Business Development |
Services
| Management and
Boards | News
and Media | Contact Us |
Home